Proteins
Proteins are biological macromolecules that play a crucial role in almost all biological processes. Within living organisms proteins are responsible for a wide variety of functions and fall into a number of important classes.
Proteins are long chain polymers built from amino acid residues. These polypeptides do not remain as loose chains, instead fold into unique three-dimensional structures. These three-dimensional structures are of key importance for the protein’s ability to carry out its biological function.
The structure of a protein is divided into four levels:
- primary
- secondary
- tertiary
- quaternary
Primary structure of a protein
The primary structure of a protein—and one of the main ways in which they are differentiated from one another—is the sequence of amino acid residues they comprise.
The individual amino acids are joined together in a long chain via a peptide bond, which occurs between the amine terminus of one amino acid and the carboxyl terminus of the other.
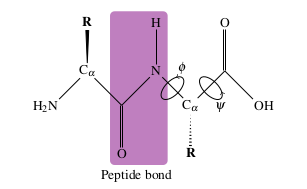
The bond is formed via the loss of one water molecule. The peptide bond is planar and relatively rigid, owing to the delocalisation of electrons between the CO and NH. The rigidity of the peptide bond has consequences for the secondary structure of a protein.
The polypeptide backbone can be described completely by the dihedral angles
Secondary structure of a protein
The secondary structure of a protein encompasses the 3D structure that the amino acid chain forms locally. Protein secondary structure elements are generally held together via hydrogen bonds interactions.
The most common types are
The polypeptide backbone can be described completely by the dihedral angles
Different combinations of these angles give rise to the different secondary structure elements:
- alpha helices]]
- beta sheets]]
- 3-10 helix
- pi-helix
One useful method for examining a protein's secondary structure is a Ramachandran plot.
Tertiary structure of a protein
The third level of protein structure is the tertiary structure, which is the overall three-dimensional structure of the protein and is vital to the function of the protein.
The dominant factor in determining the native structure of a folded protein is the thermodynamic stability of the structure. It is assumed that the native state will be the lowest energy structure.
There are a number of different ways in which the amino acids—particularly the side chains—may interact with one another, and these interactions play a role in determining and maintaining the tertiary structure. Hydrogen bonding, important in maintaining the stability of secondary structure elements, also plays a role in stabilising the tertiary structure. Also important are disulfide bonds between sulfur containing cysteine residues, which can form covalent linkages between two portions of the protein. The disulfide bonds bias the protein towards its native state and can aid in protein folding.
Hydrophobic interactions also play an important role in protein folding and formation of the tertiary structures. Residues with hydrophobic side-chains will tend to cluster together near the centre of the protein especially in aqueous environments, and this contributes to maintenance the tertiary structure.
There are three broad groups of protein tertiary structure, and all are related to the protein’s function:
- Globular proteins tend to be spherical in shape and are somewhat soluble with their hydrophobic residues in the centre. Water solubility is important for these proteins as they tend to be enzymes or transporters.
- Fibrous proteins tend to form long filaments which are largely water insoluble. These proteins are usually found in structural roles rather than regulatory, such as keratin in human hair.
- membrane proteins, which are usually responsible for transport in and out of the cell. In the case of these proteins the hydrophobic residues will usually settle within the membrane itself, and the hydrophilic residues either in the cytoplasm of the cell or in the external aqueous medium. Membrane proteins will tend to have a large concentrations of one particular secondary structure element, which sit within the membrane. Depending on the protein, they may have one or more transmembrane domains.
Protein structure is very closely related to function and misfolding of the tertiary structure can result in a number of diseases, such as Alzheimer’s or Parkinson’s disease.[1]
Quaternary structure of a protein
There is a fourth level of protein structure, the quaternary structure, which occurs when a protein comprises multiple subunits. The quaternary structure then describes the three-dimensional shape and arrangement of the subunits, and is stabilised in much the same way as the tertiary structure.
One example of a protein with quaternary structure is haemoglobin, which is made up of four haem containing subunits; haemoglobin is a heterotetramer, comprising two α subunits and two β sub units. Another example is HIV protease, which exists as a homodimer of two identical protein subunits.
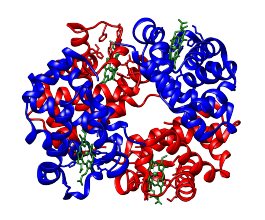
Quaternary structure of haemoglobin with α subunits shown in red, β subunits shown in blue and haem units shown in green.
Planted: Tuesday, 21 February 2023
Last tended: Monday, 23 June 2025
C.M. Dobson. Protein folding and misfolding. Nature, 426:884–890, 2003. ↩︎