Protein Secondary Structure Types
Alpha Helices
Also of great importance to this secondary structure element is the hydrogen bonding pattern.
In an α -helix the N−H of one residue is hydrogen bonded to the C − O of the residue four residues previous, giving a hydrogen bonding pattern of (i, i + 4). The combination of the dihedral angles and the hydrogen bonding pattern give rise to a helix with four residues per full 360◦ turn, meaning that the minimum length for this type of helix is four residues.
Beta Sheets
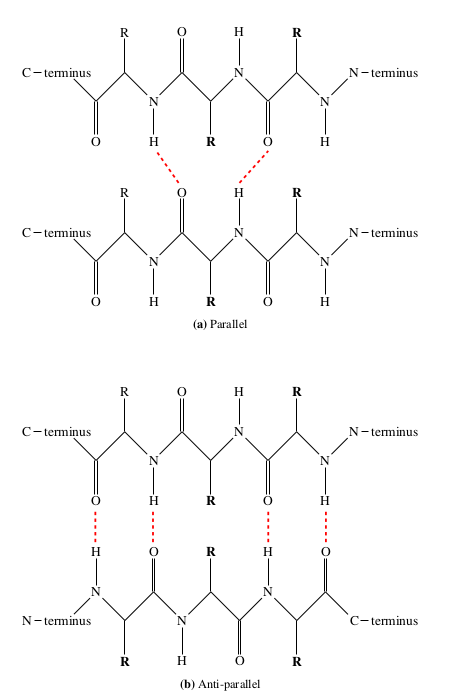
β -sheets consisting of two or more β -strands held together by inter-strand hydrogen bonds. The sheet is defined by dihedral angles of around (-135 ◦ ,135 ◦ ), though the actual values can vary greatly.
β -sheets can be described either as parallel or anti-parallel; in a parallel sheet the individual strands are aligned N-terminus to N-terminus, whereas in an anti-parallel sheet the strands are aligned N-terminus to C-terminus.
Unlike in the α -helix, the hydrogen bonds present in a β -sheet do not have to be between local or consecutive residues; in fact β -sheets are commonly formed from strands made up of residues throughout the amino acid chain.
3
The 3
Pi-helix
The
Planted: Tuesday, 26 March 2024
Last tended: Monday, 23 June 2025